 Peptide bond Peptide bond


 Bond formation Bond formation
Two amino acids can be covalently joined through a
peptide bond
.
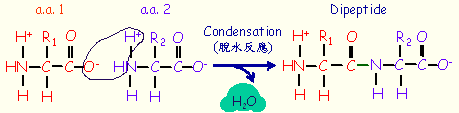
In the peptide chain, each unit is called a residue.
A peptide containing two residues is called a dipeptide, three residues is a tripeptide, etc.
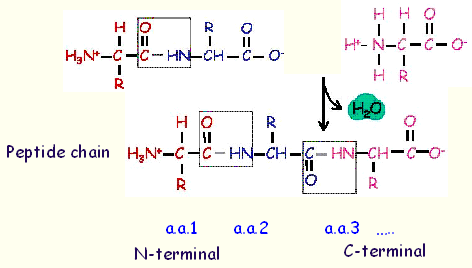
In a peptide, the amino acid residue at the end with a free amino group is called
the
amino-terminal (or N-terminal) residue
;
the residue has a free carboxyl group is called
the carboxyl-terminal (C-terminal) residue
.
The sequence of a peptide is written from its N-terminal to C-terminal end, e.g. a.a. 1-a.a.2-a.a.3...


 Property Property
The peptide C-N bond is shorter than the C-N bond in a simple amine and that the atoms associated
with the peptide bond are coplanar.
This indicated a resonance or partial sharing of two pairs of electrons between the carbonyl O and the
amide N.
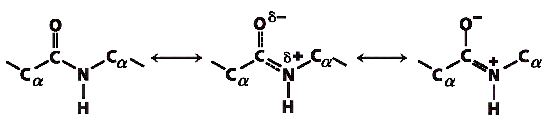
The peptide C-N bonds are unable to rotate because of their partial double-bond character.
Rotation is permitted about the
N-C
a
(
f,
phi)
, and the
C
a
-C (
y,
psi)
bonds.
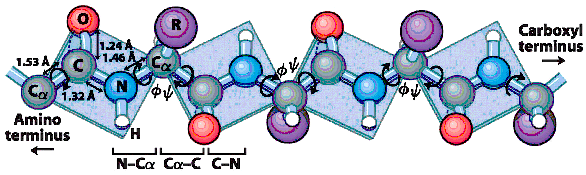
In a polypeptide chain, steric interference between atoms in the backbone and amino acid side chains
results in limited limited
f
and
y
angles.
For example, the allowed values for
f
and
y
angles for
L-Ala
residues can be graphically revealed in a
Ramachandran plot
below.
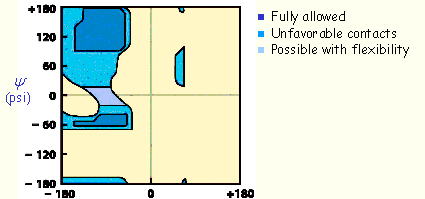
Why is the plot asymmetric?
What will the Ramachandran plot look like for other unbranched amino acids?
for branched amino acids?
What will the Ramachandran plot look like for Gly? for Pro?

 |