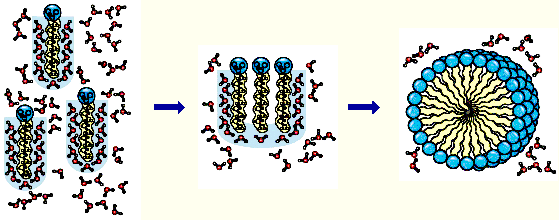
 Hydrophobic interaction Hydrophobic interaction
Hydrophobic interactions: the forces (
driven by entropy
) that hold the non-polar regions of the molecules together.
The strength of hydrophobic interactions results from the system's achieving greatest thermocdynamic
stability by
minimizing the number of ordered water molecules required to surround
hydrophobic portions of the solute molecules
.

 |